A Corrective and Preventive Action (CAPA) System is one of the most important elements of a Quality Management System.
FDA defines the purpose of CAPA System as follows
The purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and effective corrective and/or preventive action to prevent their recurrence. Verifying or validating corrective and preventive actions, communicating corrective and preventive action activities to responsible people, providing relevant information for management review, and documenting these activities are essential in dealing effectively with product and quality problems, preventing their recurrence, and preventing or minimizing device failures. One of the most important quality system elements is the corrective and preventive action subsystem.
The decision flow chart for the corrective and preventive action system as described by FDA is show below.
CAPA Decision Flow
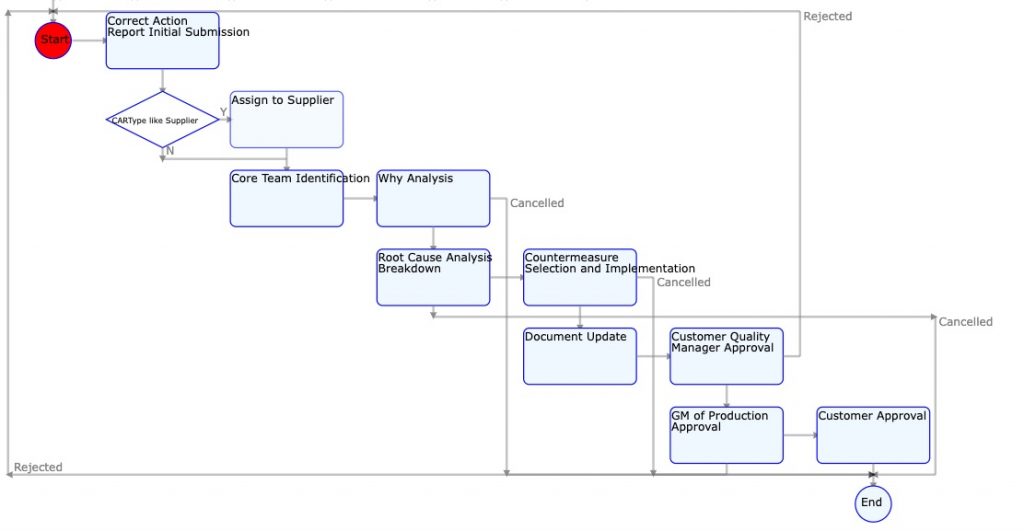
The challenge most organizations face is one of how to ensure that the CAPA decision flow and process is followed reliably for every quality event. That is where Digital Workflow driven Quality Management solutions like KaizenKit can be of great help.
Digital Workflow Based CAPA System
A digital workflow based CAPA process is ideally suited for robust, consistent, and effective CAPA subsystem of Quality Management system.